Ivd Directive 98/79/Ec Pdf . Receive an alert when an. Of 27 october 1998 on in vitro diagnostic medical. directive 98/79/ec of the european parliament and of the council. directive 98/79/ec of the european parliament and of the council. the purpose of this white paper is to provide an introduction to the european union’s ec directive 98/79/ec on in vitro diagnostic. Store all declarations on a secure server; download declaration in word or pdf format, with your logo; directive 98/79/ec of the european parliament and of the council of 27 october 1998 on in vitro diagnostic medical devices. It is therefore appropriate to. On in vitro diagnostic medical. the harmonised standard en iso 25424:2019 satisfies the requirements which it aims to cover and which are set out in directive 98/79/ec. directive 98/79/ec of the european parliament and of the council of 27 october 1998 on in vitro diagnostic medical devices.
from www.euro-chain.tw
It is therefore appropriate to. directive 98/79/ec of the european parliament and of the council of 27 october 1998 on in vitro diagnostic medical devices. directive 98/79/ec of the european parliament and of the council. the harmonised standard en iso 25424:2019 satisfies the requirements which it aims to cover and which are set out in directive 98/79/ec. On in vitro diagnostic medical. Receive an alert when an. download declaration in word or pdf format, with your logo; Of 27 october 1998 on in vitro diagnostic medical. the purpose of this white paper is to provide an introduction to the european union’s ec directive 98/79/ec on in vitro diagnostic. Store all declarations on a secure server;
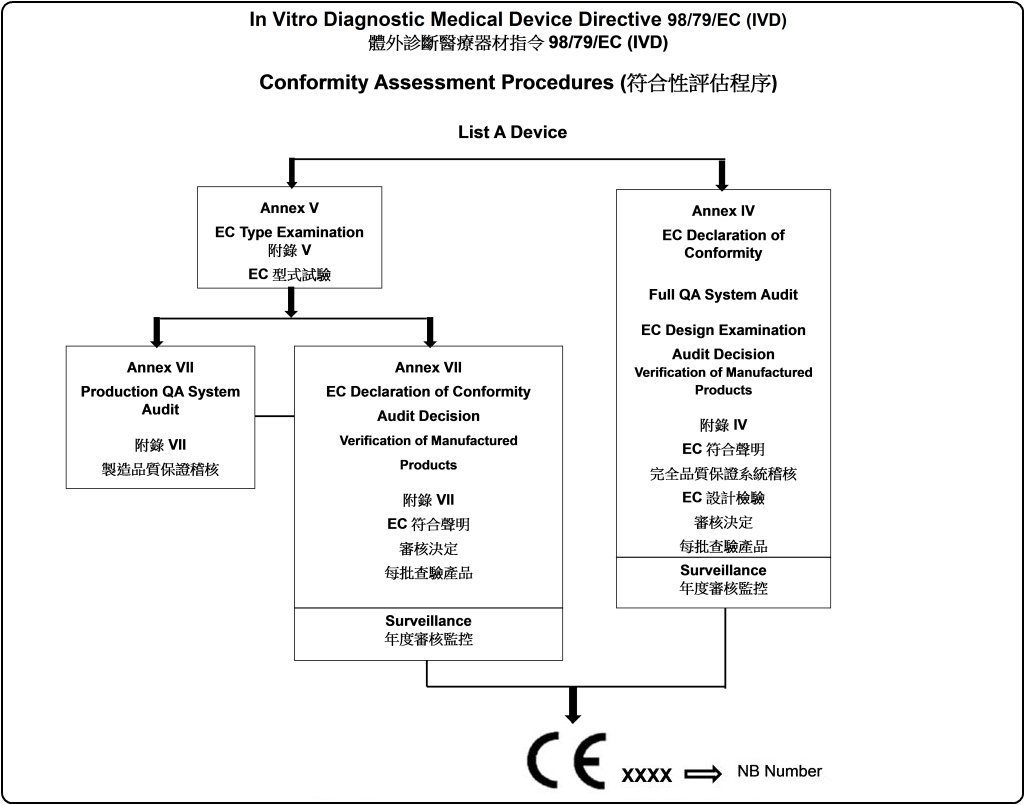
98/79/EC(IVD)
Ivd Directive 98/79/Ec Pdf the purpose of this white paper is to provide an introduction to the european union’s ec directive 98/79/ec on in vitro diagnostic. directive 98/79/ec of the european parliament and of the council. Store all declarations on a secure server; the purpose of this white paper is to provide an introduction to the european union’s ec directive 98/79/ec on in vitro diagnostic. It is therefore appropriate to. directive 98/79/ec of the european parliament and of the council. Receive an alert when an. On in vitro diagnostic medical. directive 98/79/ec of the european parliament and of the council of 27 october 1998 on in vitro diagnostic medical devices. the harmonised standard en iso 25424:2019 satisfies the requirements which it aims to cover and which are set out in directive 98/79/ec. Of 27 october 1998 on in vitro diagnostic medical. download declaration in word or pdf format, with your logo; directive 98/79/ec of the european parliament and of the council of 27 october 1998 on in vitro diagnostic medical devices.
From www.slideserve.com
PPT 歐盟對醫療器材之管理 PowerPoint Presentation, free download ID3399663 Ivd Directive 98/79/Ec Pdf directive 98/79/ec of the european parliament and of the council. download declaration in word or pdf format, with your logo; Receive an alert when an. Of 27 october 1998 on in vitro diagnostic medical. directive 98/79/ec of the european parliament and of the council of 27 october 1998 on in vitro diagnostic medical devices. the purpose. Ivd Directive 98/79/Ec Pdf.
From www.sgs.com
Understanding the EC Directive 98/79/EC on In Vitro Diagnostic Medical Devices SGS Bahrain Ivd Directive 98/79/Ec Pdf It is therefore appropriate to. On in vitro diagnostic medical. directive 98/79/ec of the european parliament and of the council. Store all declarations on a secure server; the harmonised standard en iso 25424:2019 satisfies the requirements which it aims to cover and which are set out in directive 98/79/ec. Of 27 october 1998 on in vitro diagnostic medical.. Ivd Directive 98/79/Ec Pdf.
From studylib.net
MedInfo Directive 98/79/EC on in vitro diagnostic medical devices Ivd Directive 98/79/Ec Pdf directive 98/79/ec of the european parliament and of the council of 27 october 1998 on in vitro diagnostic medical devices. directive 98/79/ec of the european parliament and of the council of 27 october 1998 on in vitro diagnostic medical devices. directive 98/79/ec of the european parliament and of the council. the harmonised standard en iso 25424:2019. Ivd Directive 98/79/Ec Pdf.
From www.euro-chain.tw
98/79/EC(IVD) Ivd Directive 98/79/Ec Pdf directive 98/79/ec of the european parliament and of the council. It is therefore appropriate to. the purpose of this white paper is to provide an introduction to the european union’s ec directive 98/79/ec on in vitro diagnostic. Of 27 october 1998 on in vitro diagnostic medical. the harmonised standard en iso 25424:2019 satisfies the requirements which it. Ivd Directive 98/79/Ec Pdf.
From slideplayer.com
European Diagnostic Manufacturers Association EQA contribution and industry expectations Dr Ivd Directive 98/79/Ec Pdf download declaration in word or pdf format, with your logo; the harmonised standard en iso 25424:2019 satisfies the requirements which it aims to cover and which are set out in directive 98/79/ec. Receive an alert when an. directive 98/79/ec of the european parliament and of the council of 27 october 1998 on in vitro diagnostic medical devices.. Ivd Directive 98/79/Ec Pdf.
From www.eclevarmedtech.com
IVD Clinical Evidence Requirements under the EU Diagnostics Regulation Ivd Directive 98/79/Ec Pdf directive 98/79/ec of the european parliament and of the council. directive 98/79/ec of the european parliament and of the council of 27 october 1998 on in vitro diagnostic medical devices. On in vitro diagnostic medical. It is therefore appropriate to. the purpose of this white paper is to provide an introduction to the european union’s ec directive. Ivd Directive 98/79/Ec Pdf.
From slideplayer.com
European Diagnostic Manufacturers Association EQA contribution and industry expectations Dr Ivd Directive 98/79/Ec Pdf the purpose of this white paper is to provide an introduction to the european union’s ec directive 98/79/ec on in vitro diagnostic. Of 27 october 1998 on in vitro diagnostic medical. download declaration in word or pdf format, with your logo; Store all declarations on a secure server; On in vitro diagnostic medical. directive 98/79/ec of the. Ivd Directive 98/79/Ec Pdf.
From www.slideserve.com
PPT «Новый подход» к технической гармонизации PowerPoint Presentation ID5178634 Ivd Directive 98/79/Ec Pdf Receive an alert when an. directive 98/79/ec of the european parliament and of the council. directive 98/79/ec of the european parliament and of the council of 27 october 1998 on in vitro diagnostic medical devices. Store all declarations on a secure server; directive 98/79/ec of the european parliament and of the council. the harmonised standard en. Ivd Directive 98/79/Ec Pdf.
From slideplayer.com
Verification of precision and bias ppt video online download Ivd Directive 98/79/Ec Pdf directive 98/79/ec of the european parliament and of the council of 27 october 1998 on in vitro diagnostic medical devices. Of 27 october 1998 on in vitro diagnostic medical. the harmonised standard en iso 25424:2019 satisfies the requirements which it aims to cover and which are set out in directive 98/79/ec. It is therefore appropriate to. directive. Ivd Directive 98/79/Ec Pdf.
From www.scribd.com
DX032851 Essential Requirements Checklist V4 (IVDD Annex I of IVD Directive 9879EC) PDF Ivd Directive 98/79/Ec Pdf It is therefore appropriate to. directive 98/79/ec of the european parliament and of the council of 27 october 1998 on in vitro diagnostic medical devices. Store all declarations on a secure server; Of 27 october 1998 on in vitro diagnostic medical. Receive an alert when an. On in vitro diagnostic medical. directive 98/79/ec of the european parliament and. Ivd Directive 98/79/Ec Pdf.
From www.scribd.com
EC Declaration of Conformity In accordance with Directive 98/79/EC Ivd Directive 98/79/Ec Pdf Store all declarations on a secure server; directive 98/79/ec of the european parliament and of the council. Receive an alert when an. download declaration in word or pdf format, with your logo; directive 98/79/ec of the european parliament and of the council of 27 october 1998 on in vitro diagnostic medical devices. directive 98/79/ec of the. Ivd Directive 98/79/Ec Pdf.
From www.slideserve.com
PPT CE mark issues PEI view PowerPoint Presentation, free download ID921732 Ivd Directive 98/79/Ec Pdf directive 98/79/ec of the european parliament and of the council of 27 october 1998 on in vitro diagnostic medical devices. It is therefore appropriate to. Store all declarations on a secure server; On in vitro diagnostic medical. download declaration in word or pdf format, with your logo; the purpose of this white paper is to provide an. Ivd Directive 98/79/Ec Pdf.
From present5.com
CE mark issues PEI view Micha Nübling PEI Ivd Directive 98/79/Ec Pdf It is therefore appropriate to. On in vitro diagnostic medical. directive 98/79/ec of the european parliament and of the council. Of 27 october 1998 on in vitro diagnostic medical. the harmonised standard en iso 25424:2019 satisfies the requirements which it aims to cover and which are set out in directive 98/79/ec. directive 98/79/ec of the european parliament. Ivd Directive 98/79/Ec Pdf.
From dokumen.tips
(PDF) DIRECTIVE (98/79/EC) DIAGNOSTIC MEDICAL DEVICES OFFICIAL DOKUMEN.TIPS Ivd Directive 98/79/Ec Pdf directive 98/79/ec of the european parliament and of the council. Of 27 october 1998 on in vitro diagnostic medical. It is therefore appropriate to. Receive an alert when an. directive 98/79/ec of the european parliament and of the council of 27 october 1998 on in vitro diagnostic medical devices. directive 98/79/ec of the european parliament and of. Ivd Directive 98/79/Ec Pdf.
From www.pdffiller.com
Fillable Online Directive 98/79/EC ClassificationTV SD PSB Tuv Ivd Directive 98/79/Ec Pdf directive 98/79/ec of the european parliament and of the council of 27 october 1998 on in vitro diagnostic medical devices. directive 98/79/ec of the european parliament and of the council. Of 27 october 1998 on in vitro diagnostic medical. the harmonised standard en iso 25424:2019 satisfies the requirements which it aims to cover and which are set. Ivd Directive 98/79/Ec Pdf.
From medexter.com
Medexter Healthcare Momo is declared a medical device in accordance with the EU IVD Directive Ivd Directive 98/79/Ec Pdf the purpose of this white paper is to provide an introduction to the european union’s ec directive 98/79/ec on in vitro diagnostic. download declaration in word or pdf format, with your logo; Receive an alert when an. It is therefore appropriate to. Of 27 october 1998 on in vitro diagnostic medical. directive 98/79/ec of the european parliament. Ivd Directive 98/79/Ec Pdf.
From www.euro-chain.tw
98/79/EC(IVD) Ivd Directive 98/79/Ec Pdf Store all declarations on a secure server; the purpose of this white paper is to provide an introduction to the european union’s ec directive 98/79/ec on in vitro diagnostic. It is therefore appropriate to. download declaration in word or pdf format, with your logo; directive 98/79/ec of the european parliament and of the council of 27 october. Ivd Directive 98/79/Ec Pdf.
From www.mybeckman.cn
Standardized reagents and guidelines overview Ivd Directive 98/79/Ec Pdf the harmonised standard en iso 25424:2019 satisfies the requirements which it aims to cover and which are set out in directive 98/79/ec. It is therefore appropriate to. download declaration in word or pdf format, with your logo; Store all declarations on a secure server; directive 98/79/ec of the european parliament and of the council of 27 october. Ivd Directive 98/79/Ec Pdf.